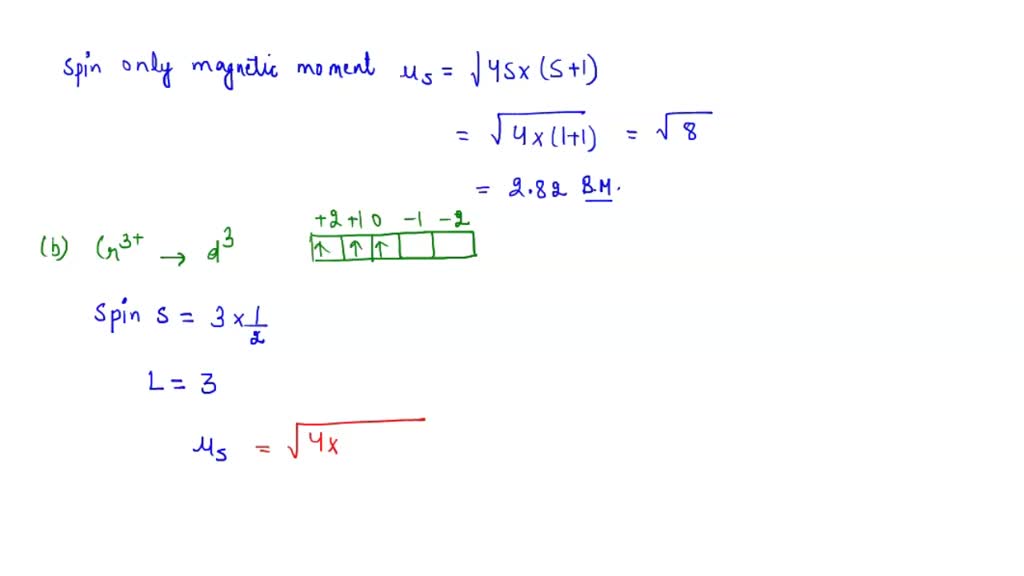
Calculate the spin only magnetic moment of La^3+. - Sarthaks eConnect | Largest Online Education Community
SOLVED: Calculate the magnetic moment of V 3+, Cr3+, Pr3+, Nd3+ according to the following instructions. (a) Consider spin-only magnetic moment in your calculation (b) Consider both spin and orbital moment in
![The spin-only magnetic moments of [Mn(CN)(6)]^(4-) and [MnBr(4)]^(2-) in Bohr Magnetons, respectively, are The spin-only magnetic moments of [Mn(CN)(6)]^(4-) and [MnBr(4)]^(2-) in Bohr Magnetons, respectively, are](https://d10lpgp6xz60nq.cloudfront.net/ss/web/618499.jpg)
The spin-only magnetic moments of [Mn(CN)(6)]^(4-) and [MnBr(4)]^(2-) in Bohr Magnetons, respectively, are
![The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` - YouTube The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` - YouTube](https://i.ytimg.com/vi/no_ZGBD689M/maxresdefault.jpg)
The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` - YouTube

The highest value of the calculated spin-only magnetic moment (in BM) among all the transition - YouTube

The spin only magnetic moment of a divalent ion in aqueous solution (atomic number 29) is ______ BM. Option: 1 2 Option: 2 - Option: 3 - Option: 4 -
![The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are](https://d10lpgp6xz60nq.cloudfront.net/ss/web/1263733.jpg)
The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are
The value of the 'spin only' magnetic moment for one of the following configurations is 2.84BM. The correct one is - Sarthaks eConnect | Largest Online Education Community